45 A two-decade detour with Drosophila
I have focused on lizards for most of my career (Chapter 12). However, I made a two-decade detour to Drosophila. This upset many colleagues, who thought I had sold out to a “model species” with a natural history restricted to milk bottles. But the inspiration for that switch evolved indirectly from my lizard work on the comparative thermal biology of lizards. So we start with lizards.
My initial steps toward Drosophila were inadvertent. In 1982, Al Bennett invited me to join a project on Australian skinks, a diverse group of lizards. Some skinks prefer and tolerate high temperatures, but others do not. Did the optimum temperatures correlate with preferred body temperatures and critical thermal maxima? Because this was a phylogenetically tight clade of lizards, we could “map” co-evolutionary changes in the thermal dependence of sprint speed and behavior on a tree (not a great tree, admittedly, but still a tree).
Al and his graduate student Henry John-Alder were spending a year in Adelaide. Before I joined them, they measured field body temperatures, critical thermal limits and preferred body temperatures in numerous species of skinks.
When I joined them, we caught a few more species before heading to Adelaide for racing. We put lizards in a temperature-controlled environmental chamber. Then I would extract a lizard and chase it down the racetrack. From the computer records (elapsed times to photocells positioned along the track), we could later compute acceleration and maximal speed. Because I measured stride length in the sand, we could also calculate stride frequency. The project was repetitive but went well. Having the Talking Heads in the background raised our spirits.
Back in the states, I began the analyses. I wanted to look at how the behavioral and physiological traits co-evolved on a phylogenetic tree. Fortunately, Joe was a colleague at the University of Washington, and we often ate lunch together. Consequently, I knew about his forthcoming – and soon to become classic – paper using independent contrasts to evaluate evolutionary correlations of traits on phylogenetic trees.
Unfortunately, we were unable to use independent contrasts because the tree we had was crude and did not have branch lengths. I wanted to develop to an alternative method, which required estimating ancestral values of traits at the nodes of branches of the tree. I asked Joe how to do this with continuous traits such as preferred body temperatures, and he suggested a squared-change algorithm. This worked beautifully. Once I calculated the nodal values, I could determine whether the change in optimal temperature from a a given node to the branch tip correlated with the equivalent change in preferred body temperature.
I was intellectually in foreign territory, and Al and I worked on the project (off and on) for several years before being ready to submit. Joe was helpful but (appropriately) critical of every step of this project: your phylogeny doesn’t even have branch lengths, you are ignoring variation within species, etc. I conceded that my data were crap and that my analyses were crap, but finally I asked Joe whether anyone had done it better. He thought for a moment and said, “No.” I cannot imagine a higher compliment.
The paper Al and I published in Evolution was the first phylogenetically based analysis in physiology and has been cited frequently. I liked what we had accomplished but wondered where to go next? I was not interested in repeating that approach with a different group of lizards. Moreover, I knew that historical studies – whether phylogenetically informed or not – were inherently descriptive. For years I’d heard Bob Paine, a colleague in my department, insist on the need for experimental studies. I knew Bob was right, but I felt that field experiments with vertebrates were neither practical nor feasible in evolutionary time. [Note: Jonathan Losos, Tom Schoener, Mike Logan, and others subsequently proved me wrong!]
OK, if I was unable to figure out how to monitor evolution in real time in the field, could I do so in the laboratory? In other words, could I try experimental evolution in the laboratory? I admired Michael Rose’s studies of laboratory natural selection on life history traits in Drosophila.” I wondered whether I use laboratory natural selection on heat tolerance or some physiological trait of some animal? I considered various taxa but settled on Drosophila*. Unfortunately, none of my colleagues at the UW were doing anything like this with flies, and I knew nothing about fly husbandry.

Perhaps more importantly, I had misgivings about working with flies. When I had first began working in physiological ecology, I argued that physiological experiments should be relevant to the natural history of the organisms being studied (Huey and Stevenson 1979). Unfortunately, the natural history of Drosophila melanogaster was largely unknown outside of a milk bottle. Consequently, when deriving experimental conditions, I knew that I would be guessing about the conditions flies might experience in nature. That bothered me. For years, I decided that I would not start working with flies until I could justify my rationale to [Harry Greene,]{https://integrativebio.utexas.edu/component/cobalt/item/4592-greene-harry?Itemid=1224} a valued friend and staunch advocate of natural history. Harry is my gold standard for ecological relevance.
Then by chance I learned that Linda Partridge (a British geneticist at University of Edinburgh, and now Profesor Dame Partridge) would be attending an international conference in Vancouver, BC. Linda had started out working with birds but soon switched to flies. More than anyone, Linda would appreciate the challenges and benefits of someone trying to switch from vertebrates to flies.
I drove up to Vancouver to talk with her. I described a simple experiment. Maintain stocks of flies at different temperatures for several years, thus letting them evolve by “laboratory natural selection,” and then measure the thermal sensivities of some physiological traits. If the stocks diverged, this demonstrated that thermal sensitivity evolved rapidly, at least in the laboratory. [Note: climate warming was not yet on the evolutionary map.]
I asked Linda whether this experiment was feasible and might be of interest to fly biologists. She said yes to both questions. In fact, she said that a year or so earlier she had set up stocks of flies at 25 °C and at 17 °, with three replicate lines each. She asked whether I wanted to study her flies.
I was stunned by her generous offer; this would save me at least a year or more of preliminary fly rearing. I replied that I would love to work with them, but confessed that I did not even know how to sex flies. Linda invited me to Edinburgh where she could teach me the basics. I got a small grant (Burroughs-Wellcome), flew to Edinburgh in 1989 and 1990, and we did the project.
Honestly, I did not like working with flies. I spent many hours hunched over a microscope, repeatedly counting and sexing thousands of minute flies. Plus I had to avoid sneezing on a plate full of flies. ‘Fly pushing’ was even more tedious for me than racing lizards. Amusingly, Linda found fly pushing to be meditative. She could and sex flies at Olympic speeds.
Our experiments went well despite my clumsiness. Importantly, we demonstrated that some aspects of thermal sensitivity can evolve rapidly in the laboratory. [Again, this result came well before climate warming became a popular research topic.]
Soon after I started working with Linda, I flew to Long Beach, California to visit my parents. I then drove to Irvine to visit Al Bennett, with whom I shared many interests and research projects. As we drove to lunch, Al said, “When we get to lunch, I have something to tell you.” I replied, “When we get to lunch, I have something to tell you.’
At lunch Al told me about a laboratory natural selection project that he had just started with Rich Lenski, a college at Irvine. They reared bacteria (E. coli) over thousands of generations at different temperatures and monitored the evolution of thermal sensitivity of fitness. I told Al about the parallel project I was doing with Linda Partridge, but with Drosophila.
Our individual abrupt transitions from reptiles to “model organisms” were synchronous but hardly coincidental. Al and I often shared our frustration with the descriptive nature of historical studies, and we were independently exploring options for experimental approaches.
Although our moves to model organisms seemed natural to us, those moves were a shock (often an “offensive” one) to the world of ectotherm physiology. Moreover, Ted Garland, Jr. – Al’s former grad student and my former postdoc – soon switched from reptiles to laboratory mice. And Martin Feder – a long-term friend to both of us – switched from salamanders to Drosophila. Our switches were not coincidental. We all talked and worked together, and we were all searching for ‘new directions’ in evolutionary physiology.
The four of us accounted for a significant fraction of the literature in physiological ecology and evolutionary physiology. However, we were criticized for ‘selling out to model organisms.’ One distinguished comparative physiologist came up to me at a meeting, grabbed me by the coat lapels, and said, “What’s this crap I hear about your switching to Drosophila?” I was taken aback but invited her to listen to two co-authored talks on flies that I was giving later at that meeting. After listening, she came up and somewhat begrudgingly acknowledged that she now understood why I switched.
My experiments with Linda launched a two-decade excursion into flies. I knew I needed to learn more about working with flies; and so, whenever I was traveling to or near another university that had top fly biologists (e.g., Bill Rice, Michael Rose, Teresa Markow), I stopped by and asked their advice on protocols and issues. Much to my delight, they welcomed me into the fly business and offered advice. I was surprised by their generosity. They actually seemed to like flies (hard for me to understand) and were interested in whatever perspective I might add to the field.
Jerry Coyne and I were graduate students together at Harvard. Jerry worked extensively with Drosophila and urged me to contact Ken Weber, who was famous for his ability to do large-scale selection experiments with flies. Ken had built an ingenious apparatus that enabled him to select on resistance of flies to ethanol vapors. He lined the inside of a cylindrical glass column with internal baffles (for flies to perch on). This column was surrounded by a larger column, with water flowing between. By pumping water through that intervening space, Ken could precisely control temperature of the column (and thus the flies). He dumped a thousand flies in the top, pumped ethanol vapors down through the internal column, and then “fractionate” flies as they passed out and fell past the baffles and out the bottom. By recording time, he could quantify ethanol resistance (time to “knockdown”) and easily select for increased ethanol resistance.
I was intrigued and wrote to Ken. I suggested that we could progressively heat water in the column until the flies were “knocked down.” By recording column temperature as the flies fell out the bottom, we could measure “knock-down” temperature for many flies in just a few minutes.
Ken thought it would work and invited me to Minnesota. He hooked a column to temperature-controlled water bath, tossed hundreds of flies into the top of the column, and began heating the water. When the temperature in the column reached about 34 °C, the flies started tumbling down and out of the column, much to our delight. In roughly the same time I had needed to measure the Critical Thermal Maximum temperature for one lizard, I could measure knock-down temperature of a thousand flies. [Ok, counting and sexing did take a few minutes more.] The appeal of flies was blatantly obvious.
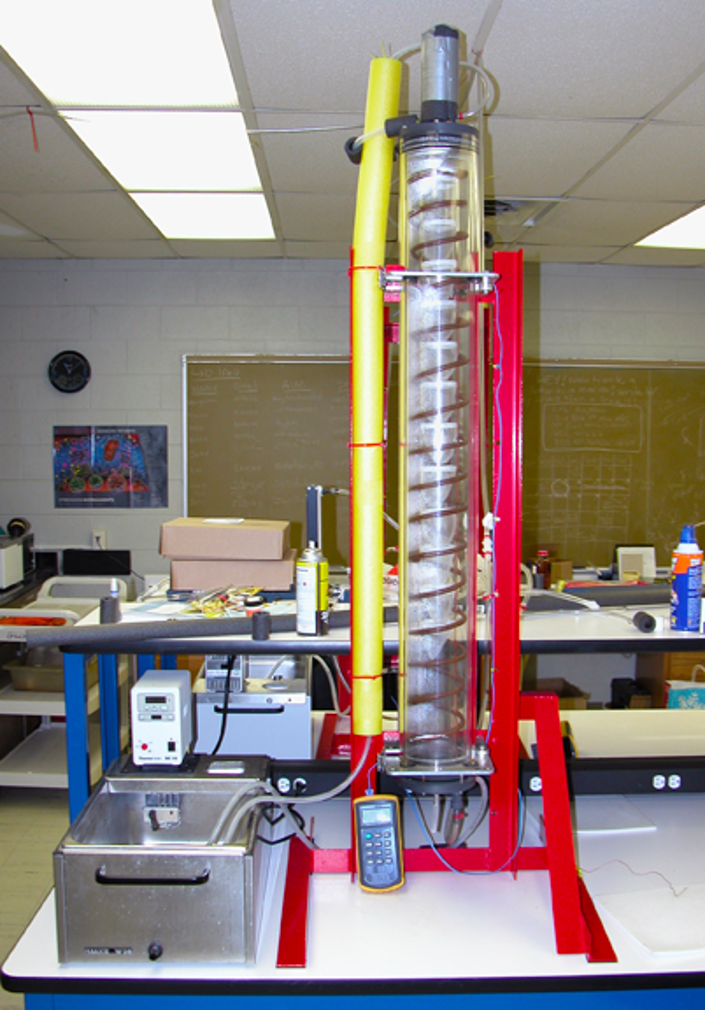
Importantly, the flies revived quickly and were not sterilized by this brief exposure to high temperature. That meant I could select on knock-down temperature.
I was awarded an NSF grant (1993) to do the study and hired George Gilchrist as the postdoc. George had been well trained in Joel Kingsolver’s lab here at the University of Washington. George was smart, an enthusiastic experimenter, and a superb programmer. George was the ideal postdoc and became a great friend. We published several papers using Ken’s column, as well as several studies on phenotypic plasticity.
Much of our initial fly work was in collaboration with Wayne Crill, a talented undergraduate. We derived a series of complex experimental designs, manipulating both the father’s and mother’s temperatures (each at 2 levels), developmental temperatures (2 levels), and adult temperatures (2 levels), with the entire experiment replicated. This hideously complex design enabled us to quantify how paternal, maternal, and developmental temperatures (as well as offspring sex) influence various traits (size, shape, color, thermal sensitivity, and egg size).
The timing for setting up crosses was complex, as we had to set up the low-temperature flies well before the high- temperature ones, so all would become adults at about the same time. Wayne was an organizational wizard – a talent I have yet to develop.
We ended up with two solid papers, showing, for example, developmental temperature and sex had (not surprisingly) effects on most traits. We also found that father’s temperature or mother’s temperature affected some traits, albeit slightly. Flies with 25 °C mothers had slightly higher knock-down temperature than did files with 18 °C mothers, and flies with 25 °C fathers had relatively longer wings. Our documentation of both within- and between-generation effects of temperature suggest that comparative and phenotypic studies need to standardize experimental environments, at least over two generations.
In addition to these studies of phenotypic plasticity, George and I used our “Weber column” to conduct an artificial selection experiment on knock-down temperature (Tkd). We had an up-selected line (the 25% of flies with the highest Tkd) and a control line (random 25%), with multiple replicates of each line. George soon noticed that the distributions of knock-down temperatures appeared bimodal, not unimodal as expected. He immediately suspected that we were inadvertently selecting on a major gene. I poo-pooed that; but after seeing the distributions for the next few generations of selection, I had to concede the point to George! We immediately modified the selection regime, selecting flies from the upper temperature mode (as before), as well as from the lower mode.
These experiments were relentless (a new round of selection with many lines every few weeks), but they worked well. We observed strong evolutionary responses. In only 20 generations of selection, the up-selected line had “lost” the lower temperature mode, and the low-mode line had “lost” the upper temperature mode! In other studies, we detected bimodality of Tkd in multiple populations of flies from three continents. Thus even though this bi-modality appeared to be worldwide, we could eliminate it in just a few generations of selection. Thermal sensitivity of flies was evolutionarily malleable, at least in the lab, as Linda Partridge and I’d found (above).
I give full credit to George Gilchrist, who tragically died of cancer in 2020. He had boundless enthusiasm not only for the experiments themselves, but also for data analyses and graphics. George was a master and a friend. We both gained respect for prior generations of fly pushers who led the way.
At this point we had shown that flies had the genetic potential to evolve rapidly in response to temperature. However, my natural history upbringings kept bothering me. Our studies were performed in the laboratory, where variables were easily controlled. However, our experiments prevented flies from using their behavior to evade heat stress. Yet in nature, behavior is often an organism’s first line of defense against any perturbation, thermal or otherwise. Perhaps our results then were not relevant to natural populations.
Consequently, I wanted to find a way to study the thermal evolution in nature. I happened across a series of paper by Antonino Prevosti, a distinguished evolutionary geneticist from Barcelona. He documented latitudinal clines in body size and also in frequencies of chromosomal inversions in Drosophila subobscura, a fly native ranging from North Africa into Scandinavia. But Prevosti and colleagues were unable to determine conclusively whether these clines were adaptive or merely a result of historical contingency.
In 1968, Chilean scientists made their annual collecting trip to Puerto Montt, a major port south of Santiago. They noticed some small black flies that had never seen in previous collections. They identified these flies as Drosophila subobscura and knew that they must have been introduced by a ship coming from Europe. They searched and subobscura only in and around the port, but by the following year they found that these flies spread quickly up and down much of coastal Chile.

In 1972, Andy Beckenback (a Canadian geneticist) discovered D. subobscura in the Pacific Northwest! Genetic evidence soon determined that the North and South American flies came from the same introduction.
Prevosti jumped at the chance to study this double invasion. Knowing that these flies had spread rapidly up and down the coasts of North and South America, he reasoned that they would be subject to clinal selection along strong latitudinal and environmental gradients that roughly paralleled those in the Old world. Would the invasive flies on both continents evolve latitudinal clines (body size, chromosomal inversions) that parallel the classical ones in Europe? If so, how fast could clines evolve?
Just a few years after the introduction, Prevosti et al. (1988) determined that latitudinal clines in chromosomal inversion frequencies were evolving and were generally in the same direction as in the Old World. This was replicated and rapid evolution on continental scales in nature! Pegueroles et al. (1995) also surveyed body size traits ten years after the introduction but could not detect any clinal trends.
When I read these papers, I jumped with excitement. Here was an opportunity to determine whether thermal sensitivity had evolved clinal patterns. Old World flies could serve as an established evolutionary baseline, and New World ones provided replicate opportunities for rapid clinal evolution.
In early 1992, I wrote to Prevosti and introduced myself. I said that I wanted to extend his studies by searching for latitudinal clines in thermal sensitivity. I asked whether he would object – I did not want to be an “American imperialist.”
Prevosti’s return letter was a delight. He acknowledged that he had of course thought of doing work along these lines but said he was about to retire. Moreover, working on New World flies was logistically difficult and expensive for geneticists in Europe. He generously encouraged me to go ahead and offered his help. Overall, his letter was “Old World gracious” – it could never have been written by an American.
That summer the Evolution meetings were held in Berkeley. Luis Serra was the new head of the lab, and he and Joan Balanyá were both attending the meetings. We met there, and Joan and I drove south to Gilroy (one of the Prevosti sites). Joan showed me how to collect and identify D. subobscura. Thus began a wonderful collaboration with the team from Barcelona.
A year or two later, Joan came over to the US. We collected flies along a transect from southern Canada to southern California, collecting every 15° of latitude. Joan then scored inversions and attempted to teach me how to do it. He failed. It was not his fault as my spatial sense and memory is horrible. I was untrainable. I tried – nothing I have ever tried has been harder. I could never have been a cyto-geneticist.
We measured the size of fly wings to determine whether size clines had finally started to evolve in North America. Next, Marta Pascual, Joan Bayanya, George Gilchrist, and I traveled to Chile in 1999 to run a transect. We had a fabulous trip and obtained great data on wing size and on inversions.


Finally, I traveled to Europe in 1998 and collected flies every 15° of latitude from Spain to Denmark. We now had data on wing size clines in Europe (the evolutionary baseline populations) as well as in both North and South America (the invaders). Recall that New World flies had not yet evolved clines in body size after 10 years, but clines were marked after 20 years. We described this rapid evolution on a clinal scale in Science.
We published another important paper on D. subobscura. Prevosti and his colleagues had been collecting inversion frequency data in many sites in Europe for decades, including recent samples. We looked to see whether inversions that were most common at low latitudes (presumably adapted to warm temperatures) were increasing in frequency over time, as would be expected given climate warming. Joan’s cytogenetic skills, along with George’s analytical and graphical ones, came to the fore. The frequency of low-latitude inversions was increasing in parallel with the magnitude of climate warming in Europe. Another Science paper!
My foray into fly work was motivated by a desire to learn whether flies have the genetic capacity to adapt to changing climates. In both the laboratory natural selection experiment with Linda Partridge and the artificial selection experiments with George Gilchrist, the answers were a strong yes. Our work on D. subobscura showed that evolution – even on a continental scale – can occur rapidly in nature. To me, “in nature” is critical. As mentioned above, laboratory studies generally prevent organisms from using behavior to evade or at least ameliorate stress, and they may produce phenotypes that could never survive in nature. Therefore, the observed evolutionary response to laboratory selection might bear little resemblance to what occurs in nature (see Huey and Rozenzweig, 2009.
To be honest, I never enjoyed working with flies. For me, lizard hunting is real hunting and primal. I have to outsmart a lizard, and the reverse was sometimes true. In any case, if I needed 10 lizards for an experiment, I would collect until I had 10 and then head to the laboratory. Moreover, I knew that I could survive on a diet only of lizards, if push came to shove.
In stark contrast, fly “hunting” is not hunting. With flies, one deploys numerous pails with rotting bananas and yeast, and then run a trap line from pail to pail, rapidly drop an insect net over the pail, and sweep up the files. Repeat over and over. Often tens or hundreds of flies (multiple species) are trapped in a single collecting vial. Back in a hotel room, I have to anesthetize and sort flies; only then do I learn whether I had sufficient females for my purposes. If not, I will have to reset a trapline the next day. If I do have sufficient females, I drive to the next site.
Despite my lack of enthusiasm for fly collection and experimentation, I was delighted by the results of the fly work. Seeing and documenting rapid evolution is exciting and has became relevant when climate warming emerged as a major ecological issue. Plus, I had thoroughly enjoyed the opportunity of working with Ken Weber, Linda Partridge, the great team from Barcelona (Luis Serra, Marta Pascual, and Joan Balanya), Wayne Crill, and George Gilchrist. We complemented each other, wrote some important papers, and often had fun. It was a good two decades. No regrets